In the late 1990s, an incredibly complex, versatile, and “pleiotropic” endogenous signaling system of the human body was named thanks to researchers exploring the properties of delta-9-THC, the major psychoactive compound within Cannabis Sativa. Christened the endocannabinoid system (ECS), this signaling system is one of the most diverse physiological control systems within the human body. Much still remains unknown about this integral system, as the ECS is currently not a fundamental part of the medical curriculum. This is unfortunate, as the ECS and ECS imbalance (also referred to as “clinical endocannabinoid deficiency”) is a known target of various chronic illnesses, from fibromyalgia to inflammatory bowel diseases and irritable bowel syndrome.
Today, as research continues into the mystery that is the ECS, it is imperative that physicians stay ahead of ECS research and its growing clinical applications. This evidence-based review will explore and justify the need for ECS science in medical education based on the most current peer-reviewed, clinical research, and the future of medicine as the ECS is further integrated into modern pharmacopeia.
How Was The Endocannabinoid System Discovered?
Homology cloning and radiolabeling in 1988 helped identify the first crucial element of the vast system that is the ECS: the CB1 G-protein coupled receptor. G-protein receptors are famous for their transmembrane loops, with the rhodopsin receptor (that gives us the ability to see) being the most well known. While the CB1 receptor was found to be highly present throughout the brain, the newly-discovered CB2 receptor was found more prevalently in immune cells. Through studying the dynamic effects, which the phytocannabinoids of cannabis have on the human body, the internal system of endocannabinoids was discovered.
The ECS: A Truly Unique Signaling System In More Ways Than One
Phytocannabinoids and the endogenous cannabinoids anandamide and 2-AG share similarities, but they are very unique compared to other signaling systems, such as those utilized by dopamine or serotonin. One of the most unique attributes of cannabinoids and endocannabinoids is that they utilize an entirely lipid (fat) based system of signaling. By observing that the exogenous phytocannabinoids from the Cannabis Sativa plant, such as THC and CBD, were also lipids with an incredibly hydrophobic (“water fearing” or “water repelling”) nature, the same could be hypothesized of the body’s own manner of utilizing this same signaling system. Ultimately, this led to the discovery of the aforementioned endogenous cannabinoid molecules anandamide and 2-AG (2-arachidonicglycrol), and opened up a line of study into a vast new control system known as the ECS within the human body.
Beyond CB1/CB2 Pharmacology: Phantom “Third Cannabinoid Receptors”
Eventually, researchers surmised that trying to explain all known ECS phenomena through the lens of only 2 receptors (CB1 and CB2) was insufficient, as disparities were observed. The search for non CB1/CB2 G-protein coupled receptors that play a role in the ECS began, and new discoveries (some of which we will explore in this guide) are being unearthed every year. Together, these components that constitute the ECS have been found to control a host of important human physiological functions, from memory and mood to pain control and digestion.
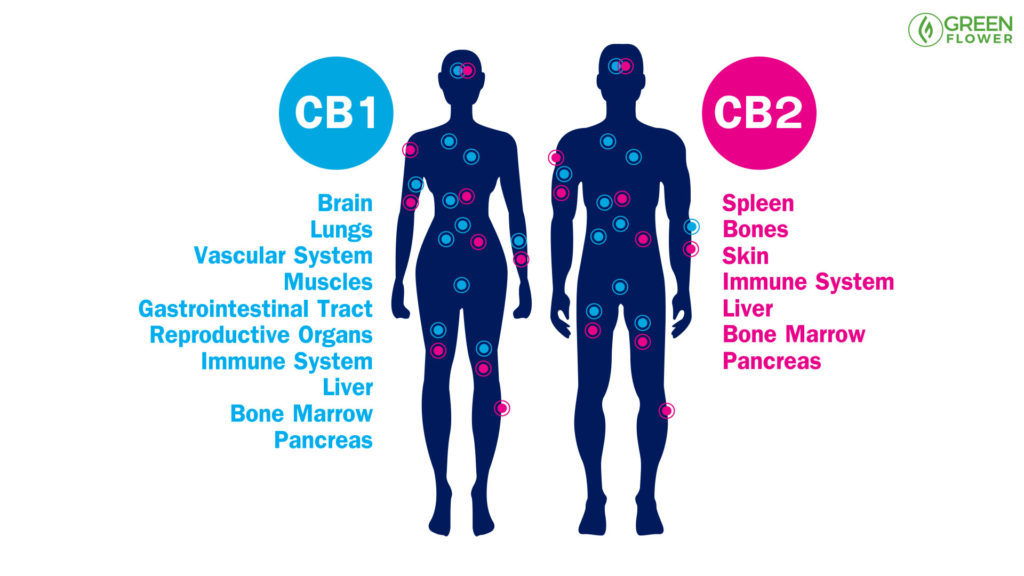
The Key Constituents Of The Endocannabinoid System’s Architecture
Three key features predominantly compose the Endocannabinoid System:
- 2 G-protein coupled cannabinoid receptors (CB1 & CB2)
- Their endogenous ligands, the two best known being anandamide and 2-arachidonoylglycerol (2-AG)
- The proteins and enzymes responsible for regulating endocannabinoid levels in the body and their action at receptors.1
It has also been established that there is endocannabinoid activity at non-CB1/CB2 receptor sites, and new discoveries of what we’ve dubbed “phantom third ‘cannabinoid’ receptors” and their functioning are being uncovered every year. For instance, vanilloid receptors are ion-gated non-G-protein coupled receptors that play a key role in nociception (pain perception), the perception of noxious substances, thermal regulation, and more. They are completely separate from endocannabinoid receptors in structure and function. However, a recent 2017 study established the interaction between cannabinoid and vanilloid systems on anxiety in male rats. The putative receptors GPR18 and GPR55 have been identified as novel endocannabinoid receptor targets in the treatment of pain, as well.5 The nature of our endogenous cannabinoids, anandamide and 2-AG being the two best-studied, is unique relative to other common neurotransmitters like dopamine, acetylcholine, GABA, or serotonin, in that they’re lipid-based. Furthermore, cannabinoids are “very promiscuous” regarding their site of action, and contradictory results pushed researchers to explain the ECS beyond simply CB1 and CB2 pharmacology. This “expanded endocannabinoid system” that extends beyond known cannabinoid receptors was dubbed in a 2019 study as the “endocannabinoidome.”
The Endocannabinoidome In Further Detail
Research has helped identify an expansive and ubiquitous signaling system that is the ECS, and it’s unique and significant in many ways. The CB1 receptor, of which delta-9-THC is a partial agonist, is ubiquitous in the human brain, as well as within the brains of Reus monkeys. In fact, there’s a 10-fold greater prevalence of CB1 receptors in the brain than there are opioid receptors! The endocannabinoid receptor prevalence rivals that of benzodiazepine receptors as well, and suggests that the ECS is a massively complex and integral system to human health, both physiologically and psychologically. It has also been confirmed that cannabinoid receptors, such as the CB2 receptor, interact with opioid receptors in afferent pathways. Perhaps an analogy can better explain just how complex studying a system like the ECS is compared to other systems, and the stark contrast between the complexities of studying a multi-faceted drug like cannabis compared to a single molecule drug. I like to call this my “Plinko Analogy.”

The “Plinko” Analogy To Demonstrate Endocannabinoid System’s Complexity
The TV program The Price Is Right featured a pricing game called “Plinko,” wherein contestants dropped a single red ball into a covered wall with knobs and various buckets at the bottom. Similar to a Pachinko machine, the objective was to get the ball into the middle, highest priced bucket — a difficult task, as the ball would chaotically bounce left and right due to all the knobs protruding from the walls. Observing the ball fall through this maze and ultimately land in a bucket can be equated to the study of a single-molecule drug (like hydrocodone or morphine) in the brain. It’s not easy, but it’s simple, since you’re observing a single molecule’s signaling behavior. However, with cannabis, there are over 100 known cannabinoids and several hundred terpenoids that interplay with other compounds, such as alcohol esters, to make up the unique phenotypes and chemotypes of Cannabis Sativa. To study the effects of cannabis, including the entourage effect caused by the harmonic interplay between these active and inactive compounds, imagine the game Plinko being played with hundreds of different colored balls, all representing the cannabinoids, and hundreds of smaller balls of even more various colors representing all the other compounds (e.g. terpenoids, flavonoids, esters, etc.) thought to act together to create any single strain’s phenotype and chemotype. Indeed, this is no small task, and the lens that we use to observe and perform drug design may be too limited to accommodate a multidimensional drug like cannabis. However, this should motivate us to expand our thinking and framework to a less binary and more quantum approach that allows for understanding such variability. There are new discoveries in the endocannabinoidome being unearthed every year, adding further layers of both understanding and complexity to our knowledge of this system. One thing is without doubt, however, and that is the fundamental importance of this system in human health and disease.
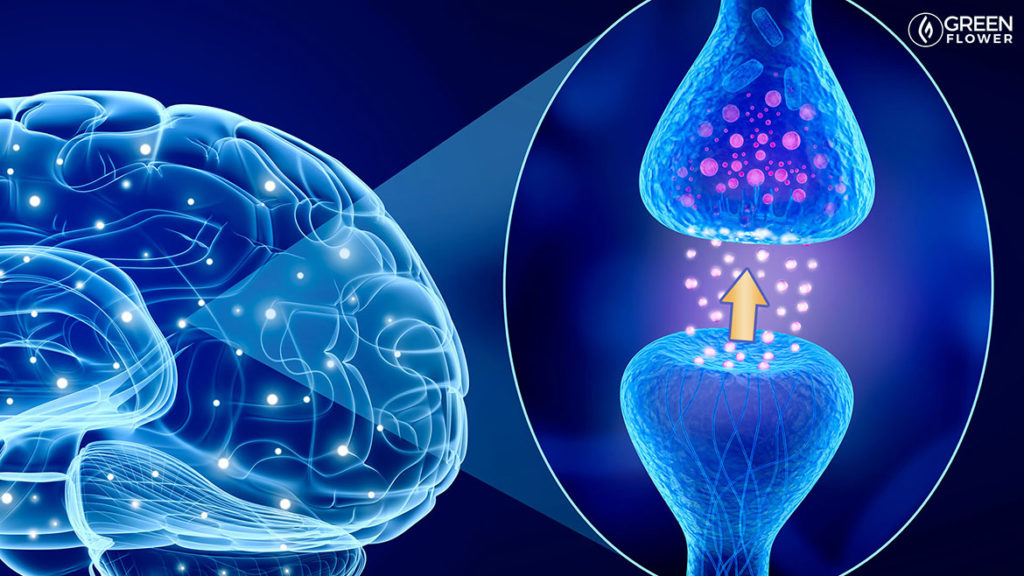
The ECS Is The Only Exclusively Retrograde Signaling System In Biology
One element left out of the Plinko analogy is that the ECS is an exclusively retrograde signaling system. That is, signals travel “backward” from the post-synaptic cleft to the pre-synaptic cleft, and the ECS is the only system known in biology to exclusively use this mode of signaling. “Lipophilic” (“fat-loving”) endocannabinoids diffuse from the post-synaptic cleft to the pre-synaptic cleft and stimulate G-protein coupled receptors. Thus, imagine the Plinko example with the balls travelling “backward” and going up the board, then binding to G-protein coupled receptors on the pre-synaptic cleft and inhibiting neurotransmitter release for “tens of seconds.” This helps establish a neuron’s ability to regulate the strength of its signaling. Therefore, “an important physiological role of endocannabinoids may be to provide a mechanism by which neurons can rapidly regulate the strength of their synaptic inputs.”9 This unique mode of signaling harkens back to both the complexity of the ECS and the need to better understand its characteristics and actions for the sake of optimizing medical care and human health.
The Future Of ECS Medicine: Synthetic Cannabinoid Drugs Are On The Horizon
It was only logical that after morphine was confirmed to modulate our endogenous opioid system, synthetic opioids would be created. The same future is highly likely, if not inevitable, when it comes to synthetic cannabinoid drugs. While phytocannabinoid-based drugs like Sativex (THC/CBD mucosal spray), Epidiolex (pure Cannabidiol oral solution), and Cannabis Sativa formulations have an incredibly high therapeutic index and have been established as safe — having never caused a fatal overdose — the same cannot be said for synthetic cannabinoid drugs! In fact, I have previously written about a synthetic cannabinoid based painkiller that caused brain death and further proved to be fatal in a clinical trial in France. Additionally, the “spice” epidemic that caused multiple fatalities is a tragic reminder of the dangers of “designer drugs.” Just because phytocannabinoids from the Cannabis Sativa plant have proven safe, haphazardly tweaking with the ECS without specificity has proved to be fatal. Although we know that cannabis is safe, that doesn’t mean the entire physiological system it acts upon, the ECS, is safe to synthetically modulate, at least not until we have a better understanding of its functioning. Since the nature of medicine will ultimately follow the natural course of creating synthetic cannabinoid drugs, the value of safety cannot be overstated.

Final Thoughts On Endocannabinoid System Exploration & Medicine
The sheer volume of research available today that demonstrates the versatility and therapeutic efficacy of cannabis warrants deeper exploration into the endogenous system upon which it acts. And, given the amount of research that supports ECS involvement in a host of important human functions and pathologies, further study of this system would benefit the entire medical field. These concepts cannot be separated, of course, and through their interplay, new cannabinoid therapies will arise. Whether new products are created by the medical and recreational cannabis industry, or pharmaceutical preparations of synthetic cannabinoids become commonplace, we cannot take the next step in medicine until we cross the frontier of the ECS. This guide only begins to explore just how important ECS functioning is to major health related systems and its crucial role in human physiology. While traditional medical curriculums leave little room for new content, I believe the significance of the ECS greatly warrants its integration into every standard medical school curriculum. I personally hope to see a day when we can begin to unlock the mysteries of the ECS and translate this newfound understanding into more optimal and efficacious therapies for people everywhere.
Works Cited
- De Petrocellis, L. & Di Marzo, V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract. Res. Clin. Endocrinol. Metab. 23, 1–15 (2009).
- Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5576607/.
- Messeguer, A., Planells-Cases, R. & Ferrer-Montiel, A. Physiology and Pharmacology of the Vanilloid Receptor. Curr. Neuropharmacol. 4, 1–15 (2006).
- Faraji, N., Komaki, A. & Salehi, I. Interaction Between the Cannabinoid and Vanilloid Systems on Anxiety in Male Rats. Basic Clin. Neurosci. 8, 129–137 (2017).
- Guerrero-Alba, R. et al. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 9, (2019).
- Cristino, L., Bisogno, T. & Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16, 9–29 (2020).
- Medicine (US), I. of, Joy, J. E., Stanley J. Watson, J. & John A. Benson, J. Cannabinoids and Animal Physiology. Marijuana and Medicine: Assessing the Science Base (National Academies Press (US), 1999).
- Role of the Cannabinoid System in Pain Control and Therapeutic Implications for the Management of Acute and Chronic Pain Episodes. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2430692/.
- Kreitzer, A. C. & Regehr, W. G. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 12, 324–330 (2002).

