Medical cannabis proponents and advocates have been heralding its ability to not only treat, but purportedly cure a variety of conditions, including cancer. However, the only FDA-approved cannabinoid therapeutics available are for the treatment of rare childhood epilepsy (see Epidiolex). Indeed, cannabis has been found to be an incredibly effective “band-aid” for a host of issues, ranging from PTSD1 to chronic pain,2 IBD,3 fibromyalgia, and many more. With so much promise and potential, one might imagine that there would be a wide variety of cannabis therapeutics on the market today. Could it be that cannabis is failing to deliver on its clinical promise to potentially revolutionize medicine? Or could it be that our current “single molecule” paradigm for researching, developing, and integrating drugs into clinical practice is simply too limited to accommodate the poorly understood and incredibly complex pharmacology of cannabis? This scientific deep dive will explore this question and make a strong case that our current framework for adopting new drugs is far too limited to accommodate cannabis, therefore leading to the lack of effective cannabinoid therapeutics currently available.
The Current State Of Medical Cannabis & Cannabinoid Therapeutics
To better assess where cannabis and cannabinoid drugs may have fallen short in clinical medicine, it is necessary to establish a clear understanding of the current state of affairs regarding these compounds. Firstly, it’s important to make a clear distinction between whole plant cannabis, the isolation of specific phytocannabinoids (such as THC or CBD), and synthetic drugs that act on the endocannabinoid system. The term medical cannabis refers to whole plant preparations or extracts of the cannabis plant, and is now available across much of the United States. Isolated cannabinoids, such as THC and CBD, have led to the FDA approval of a few notable drugs that contain these single compounds. Synthetic cannabinoid drugs still have a long way to go, however, and the few attempts at creating them have been less than successful (to say the least).4 Countless proponents of the plant have cited whole plant formulations to be far more efficacious due to the entourage effect, which is the harmonic interplay between hundreds of different molecules — from cannabinoids and terpenoids to flavonoids and esters — that are found in the cannabis plant. A 2018 study in Frontiers in Plant Science states, “the case for Cannabis synergy via the ‘entourage effect’ is currently sufficiently strong as to suggest that one molecule is unlikely to match the therapeutic and even industrial potential of Cannabis itself as a phytochemical factory.”5 While this dynamic symphony across the chemical constituents found in cannabis may indeed be profoundly therapeutic, it is this same quality, as we will soon see, that makes the potential therapeutically groundbreaking mysteries in cannabis so hard to unlock.
Our “Single Molecule” Paradigm Of Drug Discovery Is Too Limited for Cannabis
Modern drug development is rooted in a “single molecule” approach in which cannabis simply cannot integrate. In a June article I wrote, titled The Endocannabinoid System for Physicians: An Evidence-Based Review, I utilized what I’ve dubbed the “Plinko analogy” to describe the incredible pharmacological complexity of the endocannabinoid signaling system. In the Plinko challenge, a classic hallmark of the famous game show, The Price Is Right, contestants dropped a single red ball into a covered wall with knobs and various buckets at the bottom. Similar to a Pachinko machine, the objective was to get the ball into the middle, highest priced bucket — a difficult task, as the ball would chaotically bounce left and right due to all the knobs protruding from the walls. The nature of observing the red ball bounce left and right off the different knobs and ultimately land in a bucket can be equated to the study of a single-molecule drug (like azithromycin, morphine, or any other pharmaceutical). However, due to the entourage effect exhibited by cannabis, a product of over 100 known cannabinoids, hundreds of terpenoids, flavonoids, esters and more, our Plinko analogy becomes much more complex. To better illustrate just how complex, imagine the game Plinko being played with hundreds of different colored balls, all representing the cannabinoids, and hundreds of smaller balls of even more various colors representing all the other compounds (e.g. terpenoids, flavonoids, esters, etc.) thought to act together to create any single strain’s phenotype and chemotype. Clearly, some sort of quantum modeling would be required to even begin to understand how to quantify this. Unfortunately, the current paradigm for drug research and development is very much binary, and excludes novel, multi-molecule drugs from entry.
Read this scientific review I wrote for Benzinga about the entourage effect to learn more about it.
A New Way Forward: Evaluating The Potential For Multi-Molecular Modeling
The level of complexity exhibited by the cannabis sativa plant is unparalleled when compared to any and all other medicinal plants known to man. Clearly, the only way to advance cannabis therapeutics is by breaking the binary, single-molecule approach currently utilized and adopting a more versatile, quantum modeling system that can assess the interactions of multiple molecules. This concept is not completely foreign to modern pharmacology, as multiple single-molecule drugs are used in combination and then tested to evaluate their pharmacological properties and behavior. However, simply adding one drug to another significantly compounds the complexity of their combined pharmacological activity. Trying to liken this to cannabis would be like mixing many more compounds, many of which we do not fully understand the activity of, with our limited framework. This is in sharp contrast to using two or three well-studied single-molecule pharmaceutical drugs with established efficacy and safety. Nevertheless, this is likely the most logical way forward in beginning to expand the current binary framework.
New Study Evaluates “Entourage Effect” & Finds Optimal Results From Flower
While the full therapeutic potential of the entourage effect remains to be unlocked by researchers, new studies are certainly heading in the right direction. One notable 2019 study by Namdar and colleagues found that “terpenoid groups were statistically co-related to certain cannabis strains rich in Δ9–tetrahydrocannabinolic acid (THCA) or cannabidiolic acid (CBDA)” and that they were able to “enhance the activity of decarboxylase phytocannabinoids (i.e., THC or CBD).”6 More interestingly, with respect to the earlier discussion regarding the potential for cannabis to potentially exhibit anti-tumor specific cytotoxic effects (i.e. “cancer cell killing” effects), the researchers made a strong case for using whole inflorescence extracts of cannabis. “Among others, whole inflorescence extracts have been identified to alleviate chronic pain in humans7 and animals,8 enhance cytotoxic activity against cancerous cell lines,5,9 and reduce seizures in both epileptic mice10 and humans.”11 Indeed, in their sophisticated study, they found these cytotoxic effects to be enhanced by the terpene-cannabinoid combination. To give a reader an idea of just how intricately complex this system of research is compared to studying a single-molecule drug, the below diagram has been included from the research paper.
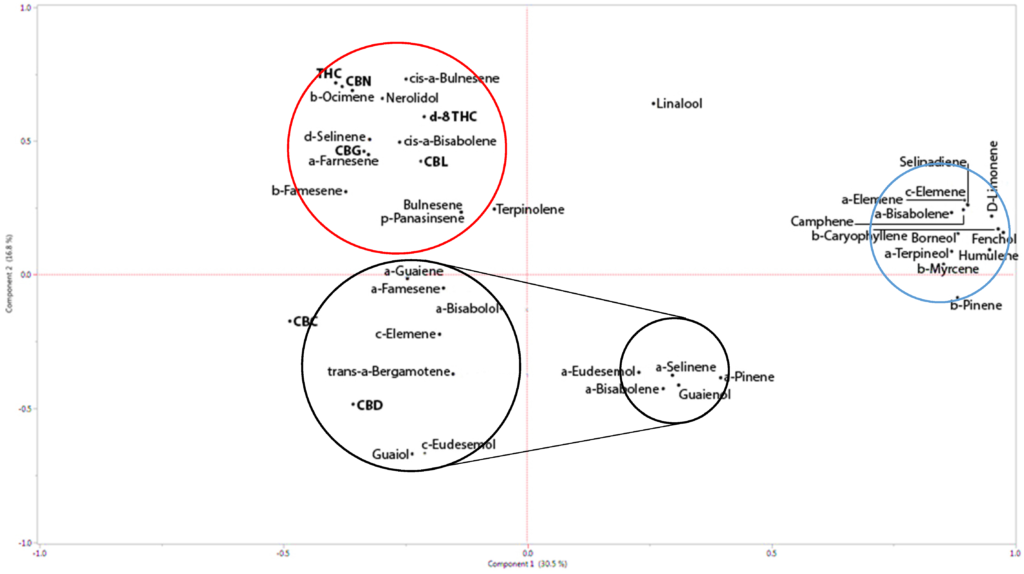
Figure 1: Principal Components Analysis (PCA) with phytocannabinoid–phytocannabinoid and phytocannabinoid–terpenoid correlations calculated for THCA and CBDA affinity.6
There May Be Hope For Cannabinoid Therapeutics, But The System Must Expand
Based on the evidence provided in this review, there may indeed be profound clinical promises hidden among the quantum complexity that is the entourage effect in cannabis. However, our current framework and stubborn refusal to change it prevents us from unearthing these potentially groundbreaking discoveries to begin with. It is the hope of this biologist and cannabis journalist that curious scientists, passionate clinicians, and the world at large continue to press forward with the drive to expand cannabinoid research and our overall understanding of the endocannabinoid system in general. Being one of the most ubiquitous and diverse signaling systems in the human body, there is a strong probability that medical cannabis does indeed hold the possibility to revolutionize medicine. However, until we can unlock the mechanisms of the vastly complex, multidimensional pharmacological system that is the entourage effect, these secrets may be forever tucked away within the inner workings of this mysterious medicinal plant.
1. Abizaid, A., Merali, Z. & Anisman, H. Cannabis: A potential efficacious intervention for PTSD or simply snake oil? J. Psychiatry Neurosci. JPN 44, 75–78 (2019).
2. Carr, D. & Schatman, M. Cannabis for Chronic Pain: Not Ready for Prime Time. Am. J. Public Health 109, 50–51 (2019).
3. Picardo, S., Kaplan, G. G., Sharkey, K. A. & Seow, C. H. Insights into the role of cannabis in the management of inflammatory bowel disease. Ther. Adv. Gastroenterol. 12, (2019).
4. France clinical trial: One person brain-dead and five in hospital after drug testing ‘accident’ in Rennes | The Independent. https://www.independent.co.uk/news/world/europe/france-clinical-trial-one-person-brain-dead-and-five-more-in-hospital-after-drug-testing-a6813791.html.
5. Russo, E. B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 9, (2019).
6. Namdar, D. et al. Terpenoids and Phytocannabinoids Co-Produced in Cannabis Sativa Strains Show Specific Interaction for Cell Cytotoxic Activity. Molecules 24, 3031 (2019).
7. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain – PubMed. https://pubmed.ncbi.nlm.nih.gov/19896326/.
8. Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. https://www.scirp.org/html/5-2500582_53912.htm.
9. Nallathambi, R. et al. Identification of Synergistic Interaction Between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis Cannabinoid Res. 3, 120–135 (2018).
10. Berman, P. et al. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 8, 14280 (2018).
11. Pamplona, F. A., da Silva, L. R. & Coan, A. C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis. Front. Neurol. 9, 759 (2018).

